SAN FRANCISCO, CA, June 26, 2019 – It was our privilege to be a part of ASM Microbe this year! We enjoyed seeing colleagues, meeting new friends, networking and learning more about the leading edge work in the ASM community. From the feedback I received, many of you were also very pleased with ASM Microbe 2019!
To those who attended our workshop, a “Clinical Perspective of the iCubate Solution for Bloodstream Infection”, thank you for making the time to be with us. The questions were thoughtful and the comments were encouraging. If you were not able to attend, please contact me for more information on the iCubate platform and assays. Those who attended ASM or are working in this field are naturally inquisitive, so let us at iCubate answer any of your questions.
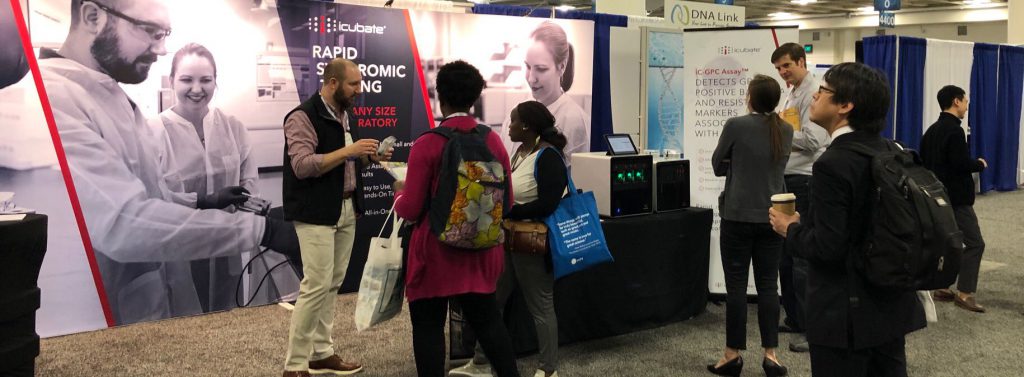
The traffic at the iCubate booth was outstanding – thanks to everyone who stopped by to see a demo, engage with us, or just learn more about what we do. While the iCubate team had a great time in San Francisco, it was good to return home to family and friends.
Keep track of iCubate by following us on social platforms where we share updates and exciting news. Look forward to seeing many of you at AACC in August. Come see iCubate at Booth 1692. Let us make our case about why iCubate is the right choice for affordable, accurate syndromic test results for laboratories of any size.

Carter Wells
CEO, iCubate
About iCubate®
iCubate is a molecular diagnostic company committed to providing rapid syndromic testing for infectious diseases leading to better care, sooner. The iCubate System is an all-in-one, easy to use multiplex PCR molecular diagnostics platform that detects and identifies bacterial and viral pathogens as well as antibiotic resistance markers for actionable results when clinicians need them. iCubate has created solutions for the detection of bloodstream infection through the iCubate platform and assays detecting gram-positive cocci (iC-GPC Assay™) and gram-negative rods (iC-GN Assay™). These assays provide identification of the most common bloodstream pathogens and clinically relevant antibiotic resistance markers to help guide antimicrobial therapy. The iC-GPC Assay™ has been cleared for clinical laboratory use by the U.S. FDA and the iC-GN Assay™ is currently under regulatory review. To learn more about iCubate, visit iCubate.com.
###
iCubate Media Contact:
Amy Mata
amy.mata@icubate.com
256-327-9568